Gut-brain axis
The microbiota–gut–brain axis is a bidirectional channel of communication between the brain and the gastrointestinal tract.The microbiota gut-brain axis (MGB) is emerging as a pivotal pathway involved in the maintenance of brain physiology as well as in the sustaining the pathophysiological events underlying neurological, neurodevelopmental, and psychiatric disorders, including mild cognitive impairment (MCI), dementia, multiple sclerosis (MS), Alzheimer’s disease (AD) and Parkinson’s disease (PD), aging, autism spectrum disorder, and depression
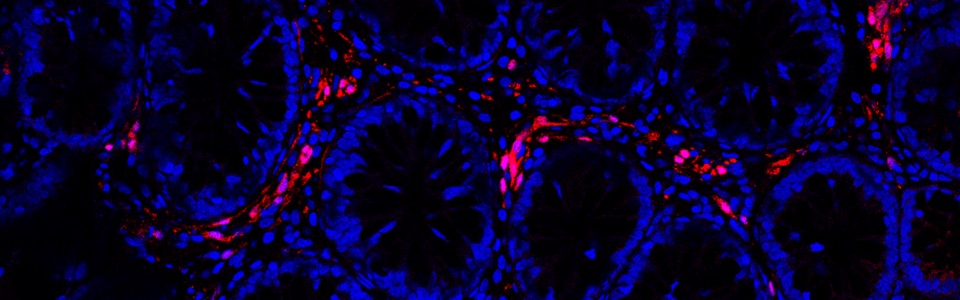
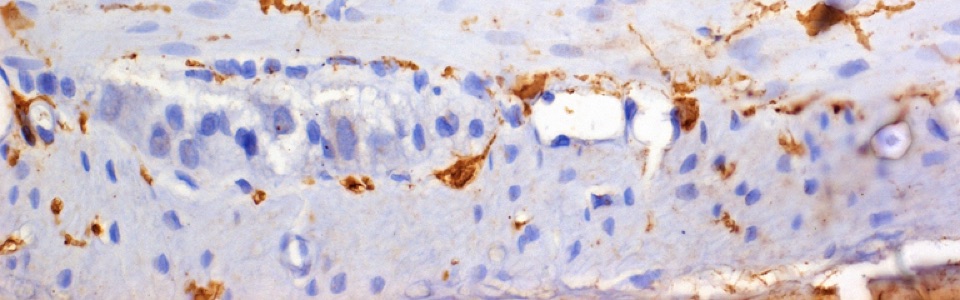
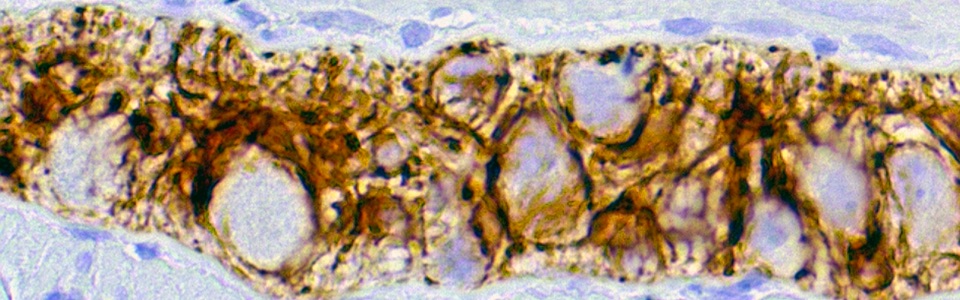
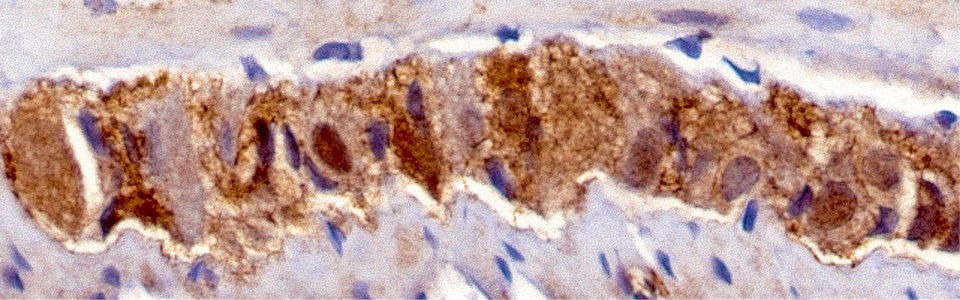
The mechanisms underlying the MGB rely mainly on interactions between the enteric bacteria, physiological barriers (including gut and blood brain barrier), immune system and nerve pathways. Indeed, pathogenic bacterial products can translocate into the blood stream and spread upwards to the brain, where they can impair blood brain barrier (BBB) integrity and influence the central circuits. In addition, bacterial products can directly activate circulating immune/inflammatory cells, which, in turn, migrate to the brain and alter BBB.
Our research investigates the role of the MGB in the disorders of the central nervous system, including neurological, neurodegenerative and psychiatric diseases. This knowledge will contribute to the design new specific therapeutical approaches (i.e. modulation of gut microbiota and drugs) to slowdown or halt disease progression, leading to the development of effective and novel therapies.
Our research focuses on three main areas:
-
Molecular mechanisms that govern MGB
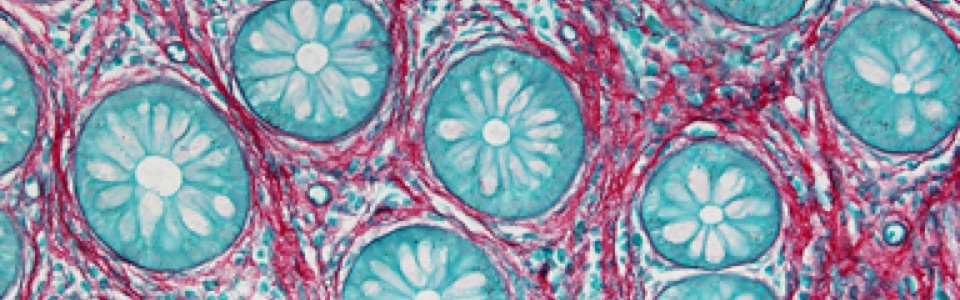
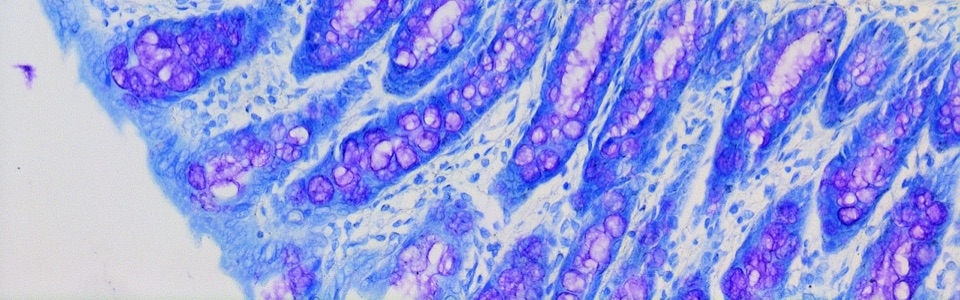
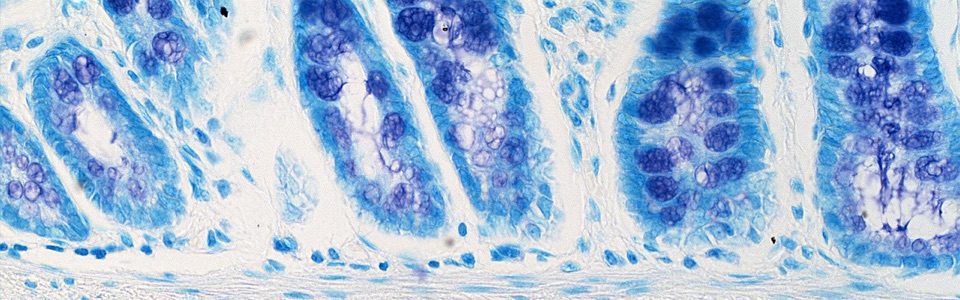
We are currently investigating the mechanisms underlying the MGB in animal models of AD and PD, including SAMP8, 5xFAD, A53T mice and 6-OHDA-treated animals. In particular, we are focused on role of gut barrier, including mucus layer, intestinal epithelial barrier and gut vascular barrier and blood brain barrier in the spreading of pathological signals from gut to the brain.
-
Novel therapeutical approaches
We ae currently characterizing the effects of pre- and probiotics and gut-directed locally acting anti-inflammatory drugs in animal models of AD and PD, including SAMP8, 5xFAD, A53T mice and 6-OHDA-treated animals. We study the effects of these therapeutical approaches on gut and brain neurogenic-immune/inflammatory responses with particular regard for NLRP3 inflammasome pathways, brain neuroinflammation and neurodegeneration and diseases cognitive and motor symptoms.
-
Translational studies
We are currently studying the role of MGB in patients with neurodegenerative diseases, including PD and AD patients. In particular, we are characterizing gut alterations in naïve patients in order to clarify the potential impact that evaluating gut alterations in brain disorders could have on clinical practice, in terms of novel diagnostic and therapeutic strategies, in the near future.
- Prokineticin-2 Is Highly Expressed in Colonic Mucosa of Early Parkinson’s Disease Patients (Movement Disorders 2024).Gabriele Bellini, Francesco Rettura, Giovanni Palermo, Chiara Ippolito, Cristina Segnani, Clarissa Pierucci, Lorenzo Fontanelli, Daniela Frosini, Vincenzo Nardini, Christian Lambiase, Nunzia Bernardini, Carolina Pellegrini, Roberto Ceravolo. Link: https://pubmed.ncbi.nlm.nih.gov/39051733/
- Enteric Glia and Brain Astroglia: Complex Communication in Health and Disease along the Gut-Brain Axis (Neuroscientist. 2023) D’Antongiovanni V, Pellegrini C, Antonioli L, Ippolito C, Segnani C, Benvenuti L, D’Amati A, Errede M, Virgintino D, Fornai M and Bernardini N) Link: https://pubmed.ncbi.nlm.nih.gov/37052336/
- The intestinal barrier in disorders of the central nervous system (Lancet Gastroenterol Hepatol. 2023) Pellegrini C, Fornai M, D’Antongiovanni V, Antonioli L, Bernardini N, Derkinderen P. Link: https://pubmed.ncbi.nlm.nih.gov/36334596/
- LRRK2 expression in normal and pathologic human gut and in rodent enteric neural cell lines (J Neurochem. 2023). de Guilhem de Lataillade A, Caillaud M, Oullier T, Naveilhan P, Pellegrini C, Tolosa E, Neunlist M, Rolli-Derkinderen M, Gelpi E, Derkinderen P. Link: https://pubmed.ncbi.nlm.nih.gov/36219522/
- Intestinal histomorphological and molecular alterations in patients with Parkinson’s disease (European Journal of Neurology 2022) Bellini G, Benvenuti L, Ippolito C, Frosini D, Segnani C, Rettura F, Pancetti A, Bertani L, D’Antongiovanni V, Palermo G, Del Prete E, Antonioli L, Nardini V, Morganti R, Pellegrini C*, Bernardini N, Ceravolo R, Fornai M and Bellini M. Link: https://pubmed.ncbi.nlm.nih.gov/36263629/
- Enteric α-synuclein impairs intestinal epithelial barrier through caspase-1-inflammasome signaling in Parkinson’s disease before brain pathology (NPJ Parkinsons Dis. 2022) Pellegrini C, D’Antongiovanni V, Miraglia F, Rota L, Benvenuti L, Di Salvo C, Testa G, Capsoni S, Carta G, Antonioli L, Cattaneo A, Blandizzi C, Colla E, Fornai M. Link: https://pubmed.ncbi.nlm.nih.gov/35022395/
- From the intestinal mucosal barrier to the enteric neuromuscular compartment: an integrated overview on the morphological changes in Parkinson’s disease (Eur J Histochem. 2021) Pellegrini C, D’Antongiovanni V, Ippolito C, Segnani C, Antonioli L, Fornai M, Bernardini N. Link: https://pubmed.ncbi.nlm.nih.gov/34802221/
- Donepezil improves vascular function in a mouse model of Alzheimer’s disease (Pharmacol Res Perspect. 2021) Pellegrini C, D’Antongiovanni V, Fornai M, Duranti E, Baldacci F, Bernardini N, Taddei S, Virdis A, Blandizzi C, Masi S, Antonioli L. Link: https://pubmed.ncbi.nlm.nih.gov/34713597/
- Palmitoylethanolamide Counteracts Enteric Inflammation and Bowel Motor Dysfunctions in a Mouse Model of Alzheimer’s Disease (Front. Pharmacol., 2021) D’Antongiovanni V, Pellegrini C, Antonioli L, Benvenuti L, Di Salvo C, Flori L, Piccarducci R, Daniele S, Martelli A, Calderone V, Martini C and Fornai M. LINK: https://pubmed.ncbi.nlm.nih.gov/34658885/
- LRRK2 is reduced in Parkinson’s disease gut (Acta Neuropathol. 2021) de Guilhem de Lataillade A, Verchere J, Oullier T, Prigent A, Durand T, Pellegrini C, Neunlist M, Baron T, Rolli‑Derkinderen M, Derkinderen P. LINK: https://pubmed.ncbi.nlm.nih.gov/34091743/
- Enteric glia at the crossroads between intestinal immune system and epithelial barrier: implications for Parkinson disease (International Journal of Molecular Sciences 2020) Benvenuti L, D’Antongiovanni V, Pellegrini C, Antonioli L, Bernardini N, Blandizzi C, Fornai M. LINK: https://pubmed.ncbi.nlm.nih.gov/33276665/
- Prodromal intestinal events in Alzheimer’s disease (AD): colonic dysmotility and inflammation are associated with enteric AD-related protein deposition (International Journal of Molecular Sciences. 2020,) Pellegrini C, Daniele S, Antonioli L, Benvenuti L, D’Antongiovanni V, Piccarducci R, Pietrobono D, Citi V, Piragine E, Flori L, Ippolito C, Segnani C, Palazon-Riquelme P, Lopez-Castejon G, Martelli A, Colucci R, Bernardini N, Trincavelli M.L., Calderone V, Martini C, Blandizzi C, Fornai M.LINK: https://pubmed.ncbi.nlm.nih.gov/32429301/
- Pathological remodelling of colonic wall following dopaminergic nigrostriatal neurodegeneration (Neurobiol Dis2020) Pellegrini C, Ippolito C, Segnani C, Dolfi A, Errede M, Virgintino D, Fornai M, Antonioli L, Garelli F, Nericcio A, Colucci R, Cerri S, Blandini F, Blandizzi C, Bernardini N. Link: https://pubmed.ncbi.nlm.nih.gov/32088380/
- High levels of beta-amyloid, tau and phospho-tau levels in red blood cells as biomarkers of neuropathology in Senescence Accelerated Mouse (Oxidative Medicine and Cellular Longevity 2019). Piccarducci R., Pietrobono D., Pellegrini C, Daniele S., Fornai M, Antonioli L., Trincavelli M.L., Blandizzi C., Martini C. Link: https://pubmed.ncbi.nlm.nih.gov/31281579/
- Constipation, deficit in colon contractions, and alpha-synuclein inclusions within the colon precede motor abnormalities and neurodegeneration in the central nervous system in a mouse model of alpha-synucleinopathy (Transl Neurodegener. 2019). Rota L., Pellegrini C, Benvenuti L., Antonioli L., Fornai M., Blandizzi C., Cattaneo A., Colla E. Link: https://pubmed.ncbi.nlm.nih.gov/30774946/
- Interplay among gut microbiota, intestinal mucosal barrier and enteric neuro-immune system: a common path to neurodegenerative diseases? (Acta Neuropathologica 2018) Pellegrini C, Antonioli L, Colucci R, Blandizzi C, Fornai M. Link: https://pubmed.ncbi.nlm.nih.gov/29797112/
- Effects of L-DOPA/benserazide co-treatment on colonic excitatory cholinergic motility and enteric inflammation following dopaminergic nigrostriatal neurodegeneration (Neuropharmacology. 2017) Pellegrini C, Antonioli L, Colucci R, Tirotta E, Gentile D, Ippolito C, Segnani C, Levandis G, Cerri S, Blandini F, Barocelli E, Ballabeni V, Bernardini N, Blandizzi C, Fornai M. Link: https://pubmed.ncbi.nlm.nih.gov/28526609/
- Intestinal dysfunction in Parkinson’s disease: Lessons learned from translational studies and experimental models (Neurogastroenterol Motil. 2016) Pellegrini C, Colucci R, Antonioli L, Barocelli E, Ballabeni V, Bernardini N, Blandizzi C, de Jonge WJ, Fornai M. Link: https://pubmed.ncbi.nlm.nih.gov/27611012/
- Alteration of colonic excitatory tachykininergic motility and enteric inflammation following dopaminergic nigrostriatal neurodegeneration (J Neuroinflammation. 2016) Pellegrini C, Fornai M, Colucci R, Tirotta E, Blandini F, Levandis G, Cerri S, Segnani C, Ippolito C, Bernardini N, Cseri K, Blandizzi C, Haskó G, Antonioli L. Link: https://pubmed.ncbi.nlm.nih.gov/27295950/
- Enteric Dysfunctions in Experimental Parkinson’s Disease: Alterations of Excitatory Cholinergic Neurotransmission Regulating Colonic Motility in Rats (J Pharmacol Exp Ther. 2016). Fornai M, Pellegrini C, Antonioli L, Segnani C, Ippolito C, Barocelli E, Ballabeni V, Vegezzi G, Al Harraq Z, Blandini F, Levandis G, Cerri S, Blandizzi C, Bernardini N, Colucci R. Link: https://pubmed.ncbi.nlm.nih.gov/26582732/
- Gastric motor dysfunctions in Parkinson’s disease: Current pre-clinical evidence (Parkinsonism Relat Disord. 2015). Pellegrini C, Antonioli L, Colucci R, Ballabeni V, Barocelli E, Bernardini N, Blandizzi C, Fornai M. Link: https://pubmed.ncbi.nlm.nih.gov/26499757/